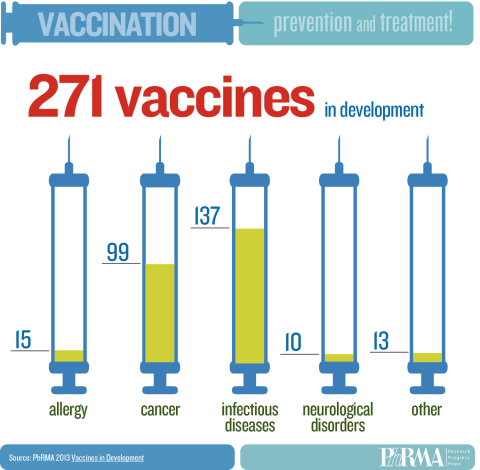
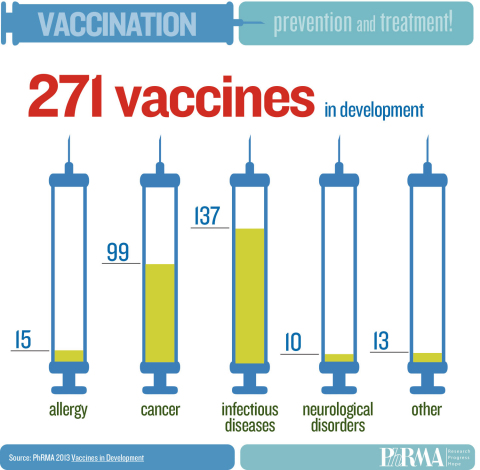
WASHINGTON--(BUSINESS WIRE)--America’s biopharmaceutical companies are currently developing 271 vaccines to prevent – and in some cases treat – a variety of conditions, including infectious diseases, various forms of cancer and neurological disorders, according to a new report by the Pharmaceutical Research and Manufacturers of America (PhRMA). The report is being released in conjunction with PhRMA’s 2013 Research and Hope Awards, which are honoring outstanding achievements in vaccine research and immunization by individuals and organizations in the medical innovation ecosystem.
For many years, vaccines have been used to successfully prevent devastating infectious diseases such as smallpox, measles and polio. According to data from the Centers for Disease Control and Prevention (CDC), 10 infectious diseases have been at least 90 percent eradicated in the United States as a result of vaccines. These innovations and subsequent immunization efforts have protected millions of children and families from needless illness.
“Biopharmaceutical research companies are working with partners across the ecosystem to apply new scientific approaches to the development of both preventative and therapeutic vaccines,” said PhRMA President and CEO John J. Castellani. “The nearly 300 vaccines in the pipeline provide great hope for protecting and improving public health in the United States and across the globe.”
The 271 vaccines in development span a wide array of diseases, and employ exciting new scientific strategies and technologies. These potential vaccines – all in human clinical trials or under review by the Food and Drug Administration (FDA) – include 137 for infectious diseases, 99 for cancer, 15 for allergies and 10 for neurological disorders. Examples include:
- A therapeutic vaccine for HIV infection intended to delay disease progression.
- A monoclonal antibody vaccine that targets both pandemic and seasonal influenza.
- A genetically-modified vaccine designed for the treatment of pancreatic cancer.
- An irradiated vaccine for protection against malaria.
Today, there are 204 active clinical trials for vaccines in the U.S., including 107 that have not yet started recruiting patients or are just now seeking volunteers to participate.
These trials, in combination with the promising new scientific approaches researchers are using, build on the successful history of vaccination against infectious diseases. For example, advances in areas such as genomics are enabling researchers to develop therapeutic vaccines, including immunotherapies for some types of cancer and other diseases. In addition, vaccines today are not limited to injectables; new delivery methods include nasal sprays, powders and transdermal applications, among others.
Some recent examples of scientific advances include an egg-free influenza vaccine that reduces development time and provides a viable treatment option for those with egg allergies, and a vaccine that is not rendered ineffective if stored at warmer temperatures, making it useful in areas where cold storage is limited.
To learn about the nearly 300 vaccines in development for both preventative and therapeutic uses, see PhRMA’s new Vaccines in Development report.
For more information on the history and public health impact of vaccines, see PhRMA’s new 2013 Vaccines Fact Book.
The Pharmaceutical Research and Manufacturers of America (PhRMA) represents the country’s leading innovative biopharmaceutical research and biotechnology companies, which are devoted to discovering and developing medicines that enable patients to live longer, healthier, and more productive lives. Since 2000, PhRMA member companies have invested approximately $550 billion in the search for new treatments and cures, including an estimated $48.5 billion in 2012 alone.
Find PhRMA Online:
- Website – http://www.phrma.org
- Facebook – www.facebook.com/PhRMA
- Blog – www.phrma.org/catalyst
- Twitter – www.Twitter.com/PhRMA and www.Twitter.com/PhRMApress
- YouTube – www.youtube.com/PhRMApress
For information on how innovative medicines save lives, visit: http://www.innovation.org
For information on the Partnership for Prescription Assistance, visit: http://www.pparx.org
For information on ensuring the flow of medicines during public health emergencies, visit http://www.rxresponse.org/