BERLIN--(BUSINESS WIRE)--Dopavision presented the primary 6-month clinical data from its proof-of-concept study of MyopiaX, a transformative approach that aims to control juvenile-onset myopia, on Friday, September 27th, 2024 at the 19th International Myopia Conference in Sanya, China. This first public presentation, delivered by Prof. Ian Flitcroft, D.Phil, FRCOphth, Coordinating Investigator of the MyopiaX-1 trial, shared data supporting MyopiaX’s safety and signals of clinical effect on myopia progression over six months.
The presentation, entitled “MyopiaX-1 6-month safety and effect outcomes on the reduction of myopia progression: A randomized, controlled, multicentre trial,” showcased data from the first and key phase of the trial, where children with myopia in Europe used either MyopiaX alone or the active control. Participants in the MyopiaX arm (n = 50) had an average change from baseline of 0.14 mm in axial length and -0.19 D in spherical equivalent refraction.
Children randomized to the active control arm (n = 34), who received spectacle lenses with defocus incorporated multiple segments (DIMS) technology, progressed 0.08 mm and -0.16 D over the same period. The MyopiaX-1 trial was not statistically powered for planned between-groups comparisons, such as a non-inferiority analysis.
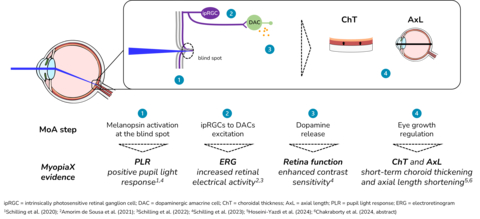
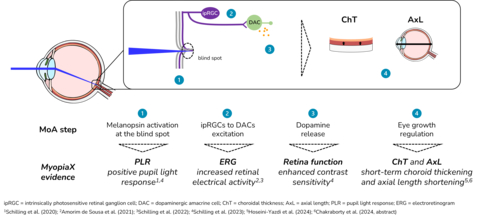
MyopiaX administers selective ocular light stimulation, targeting the blind spot, to increase melanopsin-mediated dopamine release and slow the progression of myopia. The intervention is delivered via a smartphone application, together with gaming accessories, to offer an interactive and accessible myopia control solution for children.
“MyopiaX’s selective ocular light stimulation technology is a unique approach,” said presenter Prof. Ian Flitcroft. “It holds promise as a potential new addition to our toolbox as we seek to tailor treatments to each child’s individual profile throughout their myopia progression. These initial data from the MyopiaX-1 trial help further our understanding of the role of light in myopia control.”
Mark Wuttke, CEO of Dopavision, added: “The myopia epidemic is a significant global public health challenge. We are excited about these clinical data and MyopiaX’s potential as a new tool to combat this growing issue. With MyopiaX’s excellent safety profile and the signals of clinical effect it shows on myopia progression over six months, we’re looking forward to continue to progress this program.”
Further insights from the complete MyopiaX-1 trial and learnings from this proof-of-concept will inform future clinical studies and the ongoing development of MyopiaX.
About Dopavision
Dopavision is a pioneering company in the development of innovative and accessible solutions for controlling juvenile-onset myopia. A multidisciplinary team of experts with backgrounds in biopharmaceuticals, neuroscience, ophthalmology and healthcare technology is driven by the mission to bring in a new perspective to the world of eye care. Dopavision is unique in developing products that combine selective ocular light stimulation and digital delivery methods to address the growing global problem of myopia.
Dopavision is backed by top-tier international investors, including Seventure Partners, Novartis Pharma (dRx Capital), Boehringer Ingelheim Venture Fund, and Ababax Health, and is funded by the German Federal Ministry of Education and Research (BMBF).