LONDON--(BUSINESS WIRE)--Technavio’s latest report on the global spinal trauma devices market provides an analysis of the most important trends expected to impact the market outlook from 2017-2021. Technavio defines an emerging trend as a factor that has the potential to significantly impact the market and contribute to its growth or decline.
The research study by Technavio on the global spinal trauma devices market for 2017-2021 provides a detailed industry analysis based on the product (internal fixation devices and external fixation devices), end-users (ambulatory surgical centers (ASCs), hospitals, and physician’s offices), and geography (the Americas, EMEA, and APAC).
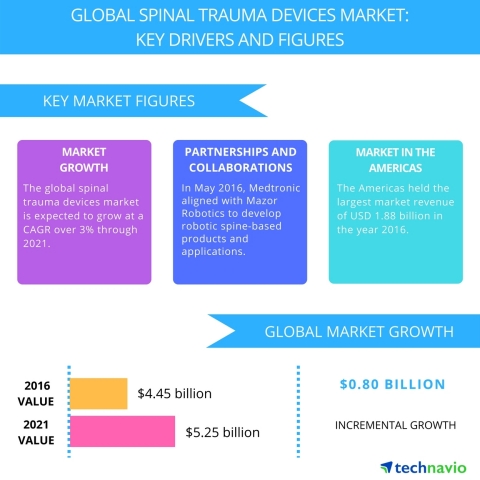
Spinal trauma refers to a severe injury to the spinal cord and requires the fixation of spinal trauma devices to treat bone deformities, facilitate bone fusion, strengthen and stabilize the spinal cord, and prevent further damage and complications. Technavio analysts forecast the global spinal trauma devices market to grow to USD 5.25 billion by 2021, at a CAGR of more than 3% over the forecast period.
Looking for more information on this market? Request a free sample report
Technavio’s sample reports are free of charge and contain multiple sections of the report including the market size and forecast, drivers, challenges, trends, and more.
The top three emerging trends driving the global spinal trauma devices market according to Technavio healthcare and life sciences research analysts are:
- Increasing partnership and collaboration activities
- Increase in volume of spinal non-fusion procedures
- Advances in treatment procedures
Increasing partnership and collaboration activities
“The spinal trauma devices vendor landscape is extremely competitive with the presence of many vendors, necessitating the formation of strategic partnerships to maintain the market share,” says Srinivas Sashidhar, a lead analyst at Technavio for orthopedics and medical devices research.
A strategic alliance can include partnership agreements, sales agreements, and distributions agreements which help the spinal trauma device manufacturers to increase their presence in new unexploited markets.
Increase in volume of spinal non-fusion procedures
Many medical facilities are suggesting patients opt for spinal non-fusion procedures over fusion procedures since they help to preserve the mobility and stability of the spine and alleviate back and leg pain while enduring heavy loads. These factors result in the better clinical outcome of the spinal non-fusion procedures, encouraging vendors to develop and introduce associated devices in the areas of artificial disc replacement, dynamic stabilization, and interspinous process decompression. The rise in the incidence of spinal disorders will also impact the number of spinal non-fusion and associated procedures.
Advances in treatment procedures
“The increase in the number of spinal trauma incidents has led to various developments in the treatment procedures. Most of the developments have taken in either robotic surgery or bone morphogenetic proteins, leading to the rising popularity of related procedures,” says Srinivas.
The robotic surgical system is a free-hand procedure, which provides highly accurate and safe minimally invasive (MI) as well as complex surgical procedures, minimizing the use of radiation during surgery. Bone morphogenic proteins (BMPs) are genetically engineered proteins, which are used for bone fusion surgery, which eliminates the need for autologous or allograft bone and all potential morbidity and limitations related to them.
The top vendors highlighted by Technavio’s research analysts in this report are:
- DePuy Synthes
- Globus Medical
- Medtronic
- Stryker
Browse Related Reports:
- Global Airway Management Products Market 2017-2021
- Global Foot and Ankle Devices Market 2017-2021
- Dental Diagnostic and Surgical Equipment Market in China 2017-2021
Become a Technavio Insights member and access all three of these reports for a fraction of their original cost. As a Technavio Insights member, you will have immediate access to new reports as they’re published in addition to all 6,000+ existing reports covering segments like cardiovascular devices, central nervous system, and in-vitro diagnostics. This subscription nets you thousands in savings, while staying connected to Technavio’s constant transforming research library, helping you make informed business decisions more efficiently.
About Technavio
Technavio is a leading global technology research and advisory company. The company develops over 2000 pieces of research every year, covering more than 500 technologies across 80 countries. Technavio has about 300 analysts globally who specialize in customized consulting and business research assignments across the latest leading edge technologies.
Technavio analysts employ primary as well as secondary research techniques to ascertain the size and vendor landscape in a range of markets. Analysts obtain information using a combination of bottom-up and top-down approaches, besides using in-house market modeling tools and proprietary databases. They corroborate this data with the data obtained from various market participants and stakeholders across the value chain, including vendors, service providers, distributors, resellers, and end-users.
If you are interested in more information, please contact our media team at media@technavio.com.