BOSTON--(BUSINESS WIRE)--Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced the planned initiation of two Phase 2 studies to evaluate the next-generation correctors VX-440 and VX-152 in triple combination regimens with tezacaftor (VX-661) and ivacaftor in people with cystic fibrosis (CF). The Phase 2 study of VX-440 is designed to evaluate the safety and efficacy of 4-week dosing of VX-440 in combination with tezacaftor and ivacaftor in approximately 40 people with CF who have one F508del mutation and one minimal function mutation and approximately 25 people with two copies of the F508del mutation. The first data from this study are expected in the second half of 2017 and are intended to support the initiation of Phase 3 development for VX-440. The Phase 2 study of VX-152 will evaluate 2 weeks of triple combination dosing in approximately 35 people with CF who have one F508del mutation and one minimal function mutation and approximately 25 people with two copies of the F508del mutation. Data from the study of VX-152 are also expected in the second half of 2017 and are intended to support the initiation of a longer-duration Phase 2b or registrational program for VX-152.
Vertex has submitted Investigational New Drug applications to the U.S. Food and Drug Administration (FDA) for both VX-440 and VX-152 and expects to start both studies by the end of 2016.
Vertex also announced today that it plans to begin Phase 1 development of an additional next-generation corrector, VX-659, by the end of 2016. The Phase 1 study is expected to enroll healthy volunteers and will also include an arm to evaluate triple combination dosing in CF patients who have one F508del mutation and one minimal function mutation. Based on data from this study, Vertex plans to start a Phase 2 study of VX-659 in the second half of 2017. The company also expects to advance a fourth next-generation corrector into Phase 1 development in 2017.
“We are committed to advancing multiple next-generation correctors in parallel to bring the best potential treatments to all people with CF who have at least one F508del mutation,” said Jeffrey Chodakewitz, M.D., Executive Vice President and Chief Medical Officer at Vertex. “We believe that the combination of a next-generation corrector with tezacaftor and ivacaftor has the potential to benefit a broad range of people with this disease, including those with minimal function mutations who do not yet have a medicine to treat the underlying cause of their CF.”
“KALYDECO and ORKAMBI are significant medical advances for many people with CF, however a very large number of our patients still do not have medicine to treat the cause of their disease,” said Patrick Flume, M.D., Director of the Medical University of South Carolina Cystic Fibrosis Center and Principal Investigator for the Phase 2 VX-152 study. “The studies announced today are a promising step forward for patients and for the treatment of this devastating disease. I look forward to working with Vertex to move these potential medicines through development and toward patients as rapidly as possible.”
The initiation of the Phase 2 studies of VX-440 and VX-152 is based on data from preclinical studies as well as recently completed Phase 1 studies for VX-440 and VX-152 that each enrolled approximately 100 healthy volunteers. Additional details for each Phase 2 study are provided below.
About the VX-440 Phase 2 Study
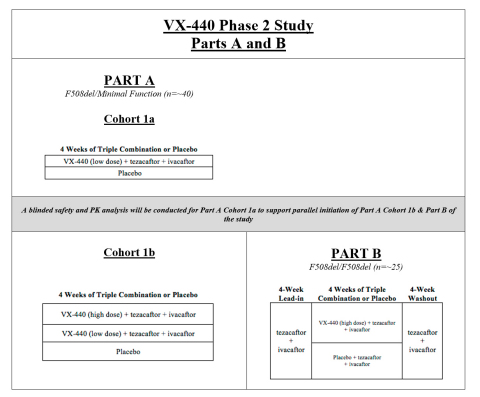
The Phase 2 study of VX-440 includes three parts. Part A of the study is designed to evaluate triple combination dosing for 4 weeks in approximately 40 patients ages 18 and older with one F508del mutation and one minimal function mutation. Part B of the study is designed to evaluate triple combination dosing for 4 weeks in approximately 25 patients ages 18 and older with two copies of the F508del mutation. A detailed study design is provided in Table 1.
Part A Cohort 1a will evaluate low-dose VX-440 in combination with tezacaftor and ivacaftor. Cohort 1a is designed to confirm that the safety and pharmacokinetic properties observed in healthy volunteers are similar in people with CF. The doses for Part A Cohort 1b and Part B will be confirmed based on review of data from Part A Cohort 1a.
The primary endpoints of the study are safety and tolerability and the absolute change in percent predicted forced expiratory volume in one second (ppFEV1). Secondary endpoints will evaluate relative improvement in ppFEV1, change in sweat chloride and change in the CF questionnaire-revised (CFQ-R) respiratory domain score, among others. Women of childbearing potential who enroll in the Phase 2 study of VX-440 will be required to use pre-specified, non-hormonal methods of contraception.
Pending completion of enrollment, Vertex expects to report data from both cohorts of Part A and from Part B in the second half of 2017. The initiation of Phase 3 development is pending data from Parts A and B and discussions with regulatory authorities.
A third part of the study (Part C) is designed to evaluate triple combination dosing for 12 weeks in approximately 130 patients ages 12 and older with one F508del mutation and one minimal function mutation. Part C will be initiated based on data from Part A and Part B and would run in parallel with potential Phase 3 development. Part C is intended to generate 12-week safety and efficacy data and to demonstrate the contribution of the components to the overall effect of the triple combination regimen.
About the VX-152 Phase 2 Study
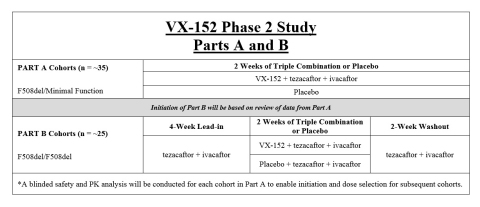
The Phase 2 study of VX-152 includes two parts. Part A of the study includes up to three dose escalation cohorts and is designed to evaluate triple combination dosing for 2 weeks in approximately 35 patients ages 18 and older with one F508del mutation and one minimal function mutation. Part B of the study includes up to two cohorts and is designed to evaluate triple combination dosing for 2 weeks in approximately 25 patients ages 18 and older with two copies of the F508del mutation. A detailed study design is provided in Table 2.
The primary endpoint of the study is safety and tolerability. Secondary endpoints will evaluate absolute and relative improvement in ppFEV1, change in sweat chloride, and change in the CFQ-R respiratory domain score, among others. Part A Cohort 1a, will evaluate low-dose VX-152 in combination with tezacaftor and ivacaftor. The doses for Part A Cohort 1b, Cohort 1c and Part B will be based on review of data from prior cohorts.
Pending completion of enrollment, Vertex expects to report data from Parts A and B of this study in the second half of 2017. These data are intended to support the initiation of a longer-duration Phase 2b or registrational program.
Advancing Additional Next-Generation Correctors into Phase 1
As part of Vertex’s commitment to find new medicines to treat the cause of CF for all people with the disease, the company is evaluating multiple next-generation correctors in parallel, both in research and development, to maximize the probability of rapidly advancing the best regimens to help the greatest number of people with CF. Consistent with that strategy, Vertex today announced that it plans to begin clinical development of a third next-generation corrector, VX-659, in 2016.
In human bronchial epithelial (HBE) cells with multiple different CFTR genotypes, including cells with two copies of the F508del mutation and cells with one copy of the F508del mutation and one copy of a mutation known to result in minimal CFTR function, the triple combination of VX-659, tezacaftor and ivacaftor exhibited higher maximal efficacy (as measured by percent of normal chloride transport) and enhanced potency compared to other next-generation correctors in triple combination.
A Phase 1 study of VX-659 is expected to begin by the end of 2016 and will evaluate single ascending doses, multiple ascending doses and triple combination dosing with tezacaftor and ivacaftor in healthy volunteers. The Phase 1 study will also include an arm to evaluate triple combination, or placebo, dosing in people with CF who have one copy of the F508del mutation and one copy of a minimal function mutation. Pending results of this study, Vertex plans to initiate a Phase 2 study to evaluate VX-659 in people with CF in the second half of 2017.
In addition to VX-659, Vertex expects to move a fourth next-generation corrector from research into clinical development in 2017.
INDICATION AND IMPORTANT SAFETY INFORMATION FOR KALYDECO® (ivacaftor)
KALYDECO (ivacaftor) is a prescription medicine used for the treatment of cystic fibrosis (CF) in patients age 2 years and older who have one of the following mutations in their CF gene: G551D, G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N, S549R, or R117H. KALYDECO is not for use in people with CF due to other mutations in the CF gene. KALYDECO is not effective in patients with CF with two copies of the F508del mutation (F508del/F508del) in the CF gene. It is not known if KALYDECO is safe and effective in children under 2 years of age.
Patients should not take KALYDECO if they are taking certain medicines or herbal supplements such as: the antibiotics rifampin or rifabutin; seizure medications such as phenobarbital, carbamazepine, or phenytoin; or St. John’s wort.
Before taking KALYDECO, patients should tell their doctor if they: have liver or kidney problems; drink grapefruit juice, or eat grapefruit or Seville oranges; are pregnant or plan to become pregnant because it is not known if KALYDECO will harm an unborn baby; and are breastfeeding or planning to breastfeed because is not known if KALYDECO passes into breast milk.
KALYDECO may affect the way other medicines work, and other medicines may affect how KALYDECO works. Therefore the dose of KALYDECO may need to be adjusted when taken with certain medications. Patients should especially tell their doctor if they take antifungal medications such as ketoconazole, itraconazole, posaconazole, voriconazole, or fluconazole; or antibiotics such as telithromycin, clarithromycin, or erythromycin.
KALYDECO can cause dizziness in some people who take it. Patients should not drive a car, use machinery, or do anything that needs them to be alert until they know how KALYDECO affects them. Patients should avoid food containing grapefruit or Seville oranges while taking KALYDECO.
KALYDECO can cause serious side effects including:
High liver enzymes in the blood have been reported in patients receiving KALYDECO. The patient’s doctor will do blood tests to check their liver before starting KALYDECO, every 3 months during the first year of taking KALYDECO, and every year while taking KALYDECO. For patients who have had high liver enzymes in the past, the doctor may do blood tests to check the liver more often. Patients should call their doctor right away if they have any of the following symptoms of liver problems: pain or discomfort in the upper right stomach (abdominal) area; yellowing of their skin or the white part of their eyes; loss of appetite; nausea or vomiting; or dark, amber-colored urine.
Abnormality of the eye lens (cataract) has been noted in some children and adolescents receiving KALYDECO. The patient’s doctor should perform eye examinations prior to and during treatment with KALYDECO to look for cataracts. The most common side effects include headache; upper respiratory tract infection (common cold), which includes sore throat, nasal or sinus congestion, and runny nose; stomach (abdominal) pain; diarrhea; rash; nausea; and dizziness.
These are not all the possible side effects of KALYDECO.
Please click here to see the full Prescribing Information for KALYDECO (ivacaftor).
INDICATION AND IMPORTANT SAFETY INFORMATION FOR ORKAMBI® (lumacaftor/ivacaftor) TABLETS
ORKAMBI is a prescription medicine used for the treatment of cystic fibrosis (CF) in patients age 6 years and older who have two copies of the F508del mutation (F508del/F508del) in their CFTR gene. ORKAMBI should only be used in these patients. It is not known if ORKAMBI is safe and effective in children under 6 years of age.
Patients should not take ORKAMBI if they are taking certain medicines or herbal supplements, such as: the antibiotics rifampin or rifabutin; the seizure medicines phenobarbital, carbamazepine, or phenytoin; the sedatives/anti-anxiety medicines triazolam or midazolam; the immunosuppressant medicines everolimus, sirolimus, or tacrolimus; or St. John’s wort.
Before taking ORKAMBI, patients should tell their doctor if they: have or have had liver problems; have kidney problems; have had an organ transplant; are using birth control (hormonal contraceptives, including oral, injectable, transdermal or implantable forms). Hormonal contraceptives should not be used as a method of birth control when taking ORKAMBI. Patients should tell their doctor if they are pregnant or plan to become pregnant (it is unknown if ORKAMBI will harm the unborn baby) or if they are breastfeeding or planning to breastfeed (it is unknown if ORKAMBI passes into breast milk).
ORKAMBI may affect the way other medicines work and other medicines may affect how ORKAMBI works. Therefore, the dose of ORKAMBI or other medicines may need to be adjusted when taken together. Patients should especially tell their doctor if they take: antifungal medicines such as ketoconazole, itraconazole, posaconazole, or voriconazole; or antibiotics such as telithromycin, clarithromycin, or erythromycin.
When taking ORKAMBI, patients should tell their doctor if they stop ORKAMBI for more than 1 week as the doctor may need to change the dose of ORKAMBI or other medicines the patient is taking. It is unknown if ORKAMBI causes dizziness. Patients should not drive a car, use machinery, or do anything requiring alertness until the patient knows how ORKAMBI affects them.
ORKAMBI can cause serious side effects including:
High liver enzymes in the blood, which can be a sign of liver injury, have been reported in patients receiving ORKAMBI. The patient’s doctor will do blood tests to check their liver before they start ORKAMBI, every three months during the first year of taking ORKAMBI, and annually thereafter. The patient should call the doctor right away if they have any of the following symptoms of liver problems: pain or discomfort in the upper right stomach (abdominal) area; yellowing of the skin or the white part of the eyes; loss of appetite; nausea or vomiting; dark, amber-colored urine; or confusion.
Respiratory events such as shortness of breath or chest tightness were observed in patients when starting ORKAMBI. If a patient has poor lung function, their doctor may monitor them more closely when starting ORKAMBI.
An increase in blood pressure has been seen in some patients treated with ORKAMBI. The patient’s doctor should monitor their blood pressure during treatment with ORKAMBI.
Abnormality of the eye lens (cataract) has been noted in some children and adolescents receiving ORKAMBI and ivacaftor, a component of ORKAMBI. For children and adolescents, the patient’s doctor should perform eye examinations prior to and during treatment with ORKAMBI to look for cataracts.
The most common side effects of ORKAMBI include: shortness of breath and/or chest tightness; upper respiratory tract infection (common cold), including sore throat, stuffy or runny nose; gastrointestinal symptoms including nausea, diarrhea, or gas; rash; fatigue; flu or flu-like symptoms; increase in muscle enzyme levels; and irregular, missed, or abnormal menstrual periods and heavier bleeding.
Please click here to see the full Prescribing Information for ORKAMBI.
About Vertex
Vertex is a global biotechnology company that aims to discover, develop and commercialize innovative medicines so people with serious diseases can lead better lives. In addition to our clinical development programs focused on cystic fibrosis, Vertex has more than a dozen ongoing research programs aimed at other serious and life-threatening diseases.
Founded in 1989 in Cambridge, Mass., Vertex today has research and development sites and commercial offices in the United States, Europe, Canada and Australia. For six years in a row, Science magazine has named Vertex one of its Top Employers in the life sciences. For additional information and the latest updates from the company, please visit www.vrtx.com.
Investor Conference Call and Webcast
Vertex will host a conference call and webcast today at 4:30 p.m. ET to review its third quarter financial results and also discuss the clinical development plans for VX-440, VX-152 and VX-659. To access the call, please dial (866) 501-1537 (U.S.) or +1 (720) 545-0001 (International). The conference call will be webcast live and a link to the webcast can be accessed through Vertex's website at www.vrtx.com in the "Investors" section under "Events and Presentations." To ensure a timely connection, it is recommended that users register at least 15 minutes prior to the scheduled webcast. An archived webcast will be available on the company's website.
Special Note Regarding Forward-looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, including, without limitation, Dr. Chodakewitz's statements in the fourth paragraph of the press release, Dr. Flume’s statements in the fifth paragraph of the press release and statements regarding the expected timing and clinical trial designs for planned clinical studies of VX-440, VX-152, VX-659 and any additional next-generation corrector. While Vertex believes the forward-looking statements contained in this press release are accurate, these forward-looking statements represent the company's beliefs only as of the date of this press release and there are a number of factors that could cause actual events or results to differ materially from those indicated by such forward-looking statements. Those risks and uncertainties include, among other things, that data from the company's development programs may not support further development or registration of VX-440, VX-152 or VX-659 (including combination regimens containing such compounds) or its other compounds due to safety, efficacy or other reasons, and other risks listed under Risk Factors in Vertex's annual report and quarterly reports filed with the Securities and Exchange Commission and available through the company's website at www.vrtx.com. Vertex disclaims any obligation to update the information contained in this press release as new information becomes available.
(VRTX-GEN)