BILLERICA, Mass.--(BUSINESS WIRE)--SRS Medical Systems, Inc., today announced that it has received CE mark approval for use of its Spanner® Temporary Prostatic Stent across the European Economic Area (EEA). The Spanner is an alternative to both indwelling (Foley) and intermittent urinary catheters for male patients with bladder outlet obstruction (BOO).
“The Spanner is proven to have significant impact on medical outcomes, and often has a transformational impact on patient quality of life,” said Lee Brody, CEO of SRS Medical. “We are excited to receive this approval, and we look forward to working with our European partners to deliver The Spanner to the patients that will benefit from it the most.”
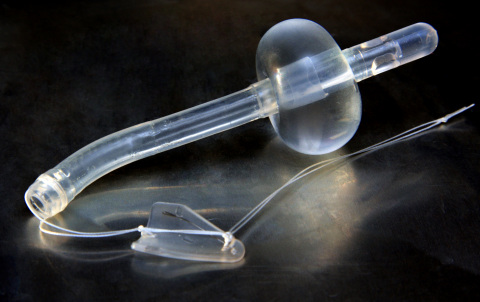
The Spanner is a completely internal device that alleviates BOO while maintaining continence. It allows patients to naturally fill and empty their bladders, often without device awareness. The Spanner has many clinical applications for treating BOO, including:
- Relieving lower urinary tract symptoms (LUTS) in patients in temporary urinary retention, including those recovering from surgical procedures;
- Reducing infection risk and medical complication in patients awaiting procedures for benign prostatic hyperplasia (BPH);
- Offering long-term symptom relief for patients unfit for prostate procedures; and
- Predicting BPH procedure outcomes in high-risk surgical candidates.
In the United States, The Spanner is approved by the Food and Drug Administration (FDA) for a single 30-day period in a limited patient population. In January 2016, SRS Medical received FDA investigational device exemption (IDE) approval for a confirmatory safety study to expand the labeling of The Spanner.
The CE mark is a result of notified body transfer to Intertek Semko AB.
About The Spanner Stent
The Spanner Temporary Prostatic
Stent is an FDA-approved Class III medical device intended to maintain
urine flow and allow for voluntary urination in certain male patients
experiencing LUTS. The device consists of two anchors and a silicone
tube that reduces resistance in the bladder neck and prostatic urethra
without stenting the external sphincter. The Spanner is placed blindly
without anesthesia, in a procedure similar to the placement of a Foley
catheter. The Spanner is reimbursed under CPT Code 53855, and is the
only prostate stent on the U.S. market.
About SRS Medical
SRS Medical is dedicated to improving the
health and well-being of men experiencing LUTS. The company, based in
Billerica, Mass., does this by delivering innovative, cost-effective
tools to urologists, enabling them to better diagnose and treat these
patients. For more information about SRS Medical: http://www.srsmedical.com.