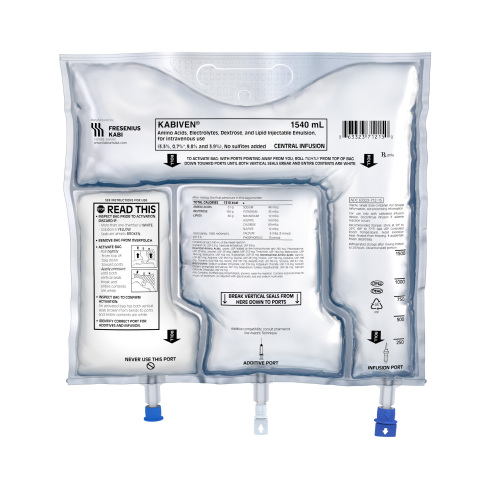
LAKE ZURICH, Ill.--(BUSINESS WIRE)--Fresenius Kabi announced today it has launched Kabiven® (amino acids, electrolytes, dextrose and lipid injectable emulsion) for intravenous use, with immediate availability. Kabiven is a parenteral nutrition (PN) product in a three-chamber bag – the only container of its kind approved in the United States. Fresenius Kabi is a global health care company that specializes in life-saving medicines and technologies for infusion, transfusion and clinical nutrition.
Kabiven was approved by the U.S. Food and Drug Administration in August. It is an intravenously infused solution of lipids, dextrose, amino acids and electrolytes in a three-chamber bag that efficiently delivers these nutrients in volumes and concentrations that meet the nutritional needs of most patients.
Fresenius Kabi expects to introduce Perikabiven® in November. Perikabiven is another Fresenius Kabi PN product that was also approved by the FDA in August. Perikabiven is intended for peripheral intravenous administration. Outside the United States, Kabiven is sold in more than 85 countries and has been used to treat millions of patients since its initial marketing authorization in 1999.
About Kabiven and Perikabiven
Kabiven and Perikabiven three-chamber bags contain amino acids with electrolytes, dextrose and lipids (Intralipid® 20%). The design of the three-chamber bag keeps the macronutrients separate and shelf-stable (without refrigeration) until the bag is activated and the contents mixed together for patient use. The formula is intended to meet the daily nutritional requirements of broad patient populations.
Kabiven and Perikabiven three chamber bags must be mixed prior to infusion. For admixing instructions see INSTRUCTIONS FOR USE in the prescribing information.
INDICATIONS AND LIMITATIONS OF USE
- Kabiven and Perikabiven are each indicated as a source of calories, protein, electrolytes and essential fatty acids for adult patients requiring parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or contraindicated. Kabiven and Perikabiven may be used to prevent essential fatty acid deficiency or treat negative nitrogen balance in adult patients.
- Kabiven is indicated for intravenous infusion into a central vein.
- Perikabiven is indicated for intravenous infusion into a peripheral or central vein.
- Neither Kabiven nor Perikabiven is recommended for use in pediatric patients < 2 years, including preterm infants because the fixed content of the formulations do not meet the nutritional requirements in this age group.
Important Safety Information
- Deaths in preterm infants have been reported in literature.
- Autopsy findings included intravascular fat accumulation in the lungs.
- Preterm and low birth weight infants have poor clearance of intravenous lipid emulsion and increased free fatty acid plasma levels following lipid emulsion infusion.
CONTRAINDICATIONS
- Known hypersensitivity to egg, soybean proteins, peanut proteins, corn or corn products, or to any of the active substances or excipients.
- Severe hyperlipidemia or severe disorders of lipid metabolism with serum triglycerides >1000 mg/dL.
- Inborn errors of amino acid metabolism.
- Cardiopulmonary instability.
- Hemophagocytic syndrome.
WARNINGS AND PRECAUTIONS
- Kabiven is hypertonic and may cause vein irritation, vein damage and even thrombosis if infused in a peripheral vein. Only infuse Kabiven into a central vein.
- Monitor for signs or symptoms of hypersensitivity reactions and discontinue infusion if reactions occur.
- Monitor patient closely for signs and symptoms of infection, hypertriglyceridemia, hyperglycemia and refeeding complications.
- Monitor laboratory parameters for alterations in electrolytes, liver and renal impairment, fluid status and coagulation parameters. Adjust rate and dose of Kabiven and Perikabiven according to clinical status.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC, Vigilance & Medical Affairs at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information, including Boxed Warning, for Kabiven and Perikabiven.
About Fresenius Kabi
Fresenius Kabi (www.fresenius-kabi.us) is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition. The company’s products and services are used to help care for critically and chronically ill patients. The company’s U.S. headquarters is in Lake Zurich, Illinois. The company’s global headquarters is in Bad Homburg, Germany. For more information about Fresenius Kabi worldwide, please visit www.fresenius-kabi.com.