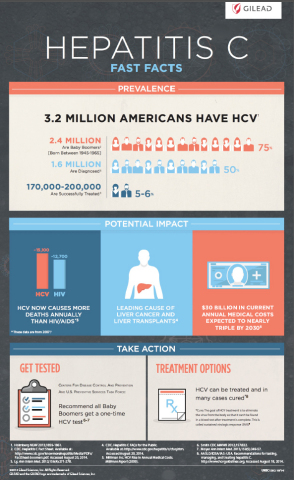
FOSTER CITY, Calif.--(BUSINESS WIRE)--Gilead Sciences, Inc. (Nasdaq: GILD) today announced that the U.S. Food and Drug Administration (FDA) has approved Harvoni® (ledipasvir 90 mg/sofosbuvir 400 mg), the first once-daily single tablet regimen for the treatment of chronic hepatitis C genotype 1 infection in adults. Harvoni combines the NS5A inhibitor ledipasvir with the nucleotide analog polymerase inhibitor sofosbuvir, approved under the tradename Sovaldi® in December 2013. Harvoni’s efficacy has been established in patients with chronic hepatitis C virus (HCV) genotype 1 infection, with a treatment duration of eight, 12 or 24 weeks depending on prior treatment history, cirrhosis status and baseline viral load. Eight weeks of treatment with Harvoni can be considered for treatment-naïve patients without cirrhosis who have baseline HCV viral load below 6 million IU/mL.
This press release has an accompanying Smart Marketing Page featuring additional multimedia and information, which can be found at: http://smp.businesswire.com/pages/us-food-and-drug-administration-approves-gileads-harvoni-ledipasvirsofosbuvir-first-once-daily.
The FDA granted Harvoni a Priority Review and Breakthrough Therapy designation, which is given to investigational medicines that may offer major advances in treatment over available therapies.
“By providing very high cure rates in as little as eight weeks and completely eliminating the need for interferon and ribavirin, which are challenging to take and tolerate, Harvoni significantly advances treatment for patients with the most common form of hepatitis C in the United States,” said Nezam Afdhal, MD, Director of Hepatology at Beth Israel Deaconess Medical Center, Professor of Medicine at Harvard Medical School and a principal investigator in the Harvoni clinical trials. “For the first time, the vast majority of patients can be cured with a once-daily pill in only eight or 12 weeks.”
Harvoni’s approval is supported by data from three Phase 3 studies, ION-1, ION-2 and ION-3. These studies evaluated eight, 12 or 24 weeks of treatment with Harvoni, with or without ribavirin, among nearly 2,000 genotype 1 HCV patients with compensated liver disease. These studies included non-cirrhotic treatment-naïve patients (ION-3), cirrhotic and non-cirrhotic treatment-naïve patients (ION-1) and cirrhotic and non-cirrhotic patients who failed prior therapy with an interferon-based regimen, including regimens containing an HCV protease inhibitor (ION-2). The primary endpoint for each study was sustained virologic response (HCV undetectable) 12 weeks after completing therapy (SVR12).
Patients who achieve SVR12 are considered cured of HCV. In these studies, ribavirin was not shown to increase response rates. Trial participants in the ribavirin-free arms (n=863) achieved SVR12 rates of 94 to 99 percent.
“Unlike other serious chronic diseases, hepatitis C can be cured and Harvoni offers patients the potential for a cure in as little as eight weeks,” said John C. Martin, PhD, Chairman and Chief Executive Officer, Gilead Sciences. “Gilead is proud to have played a role in developing a once-daily therapy that is safe, simple and well tolerated. We are now working to ensure rapid and broad access to Harvoni.”
Important Safety Information regarding warnings and precautions, adverse reactions and drug interactions is listed below. Zero percent, less than 1 percent and 1 percent of patients treated for eight, 12 and 24 weeks, respectively, discontinued treatment due to adverse events and fewer adverse events were observed in the ribavirin-free arms compared to the ribavirin-containing arms in all ION studies. The most common adverse reactions among patients treated with Harvoni (≥5 percent) were fatigue, headache, nausea, diarrhea and insomnia. For additional study details, and complete dosing information, see the Clinical Studies and Dosage and Administration sections, respectively, of the full Prescribing Information.
U.S. Patient Support Program
To
assist eligible hepatitis C patients in the United States with access to
Harvoni, Gilead has added the medicine to its Support Path™ (www.MySupportPath.com)
program. The program consists of an integrated offering of support
services for patients and providers, among them:
- Call center staffed with associates trained to help patients and their providers with insurance-related needs.
- Education and support, including a 24/7 nursing support service line.
- The Harvoni and Sovaldi Co-pay Coupon Programs, which provide co-pay assistance for eligible patients with private insurance who need assistance paying for out-of-pocket medication costs. Most patients will pay no more than $5 per co-pay.
- The Support Path Patient Assistance Program, which will provide Harvoni and Sovaldi at no charge for eligible patients with no other insurance options.
Gilead also provides support to independent non-profit organizations that provide assistance for eligible federally-insured and privately-insured patients who need help covering out-of-pocket medication costs.
To learn more about Support Path for Harvoni or Sovaldi, please visit www.MySupportPath.com or call 1-855-769-7284 between 9:00 a.m. – 8:00 p.m. Eastern, Monday through Friday.
IMPORTANT SAFETY INFORMATION
Warnings
and Precautions
Risk of Reduced Therapeutic Effect of
Harvoni Due to P-gp Inducers: Rifampin and St. John’s wort are not
recommended for use with Harvoni as they may significantly decrease
ledipasvir and sofosbuvir plasma concentrations.
Related Products Not Recommended: Harvoni is not recommended for use with other products containing sofosbuvir (Sovaldi).
Adverse Reactions
Most common (≥10%, all grades) adverse
reactions were fatigue and headache.
Drug Interactions
In addition to rifampin and St. John’s
wort, coadministration of Harvoni is also not recommended with
carbamazepine, oxcarbazepine, phenobarbital, phenytoin,
rifabutin, rifapentine, and tipranavir/ritonavir. Such coadministration
is expected to decrease the concentration of ledipasvir and sofosbuvir,
reducing the therapeutic effect of Harvoni.
Coadministration of Harvoni is not recommended with simeprevir due to increased concentrations of ledipasvir and simeprevir. Coadministration is also not recommended with rosuvastatin or co-formulated elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate due to increased concentrations of rosuvastatin and tenofovir, respectively.
Consult the full Prescribing Information for Harvoni for more information on potentially significant drug interactions, including clinical comments.
About Gilead Sciences
Gilead
Sciences is a biopharmaceutical company that discovers, develops and
commercializes innovative therapeutics in areas of unmet medical need.
The company’s mission is to advance the care of patients suffering from
life-threatening diseases worldwide. Headquartered in Foster City,
California, Gilead has operations in North and South America, Europe and
Asia Pacific.
Forward-Looking Statement
This
press release includes forward-looking statements within the meaning of
the Private Securities Litigation Reform Act of 1995 that are subject to
risks, uncertainties and other factors, including the risk that
physicians and patients may not see advantages of Harvoni over other
therapies and may therefore be reluctant to prescribe the product, and
the risk that private and public payers may be reluctant to provide
coverage or reimbursement for the product. These risks, uncertainties
and other factors could cause actual results to differ materially from
those referred to in the forward-looking statements. The reader is
cautioned not to rely on these forward-looking statements. These and
other risks are described in detail in Gilead’s Quarterly Report on Form
10-Q for the quarter ended June 30, 2014, as filed with the U.S.
Securities and Exchange Commission. All forward-looking statements are
based on information currently available to Gilead, and Gilead assumes
no obligation to update any such forward-looking statements.
U.S. Full Prescribing Information for Harvoni and Sovaldi is available at www.gilead.com.
Harvoni, Sovaldi and Support Path are trademarks or registered trademarks of Gilead Sciences, Inc., or its related companies.
For more information on Gilead Sciences, please visit the company’s website at www.gilead.com, follow Gilead on Twitter (@GileadSciences) or call Gilead Public Affairs at 1-800-GILEAD-5 or 1-650-574-3000.