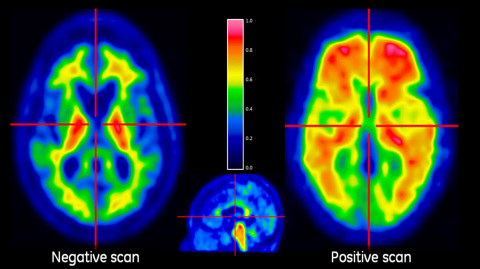
CHALFONT ST. GILES, England--(BUSINESS WIRE)--GE Healthcare announced today that VIZAMYL™ (flutemetamol (18F) solution for injection) has received marketing authorisation from the European Commission as a radiopharmaceutical medicinal product indicated for Positron Emission Tomography (PET) imaging of beta amyloid neuritic plaque density in the brains of adult patients with cognitive impairment who are being evaluated for Alzheimer’s disease (AD) and other causes of cognitive impairment. VIZAMYL™ is the only PET imaging tracer for detection of amyloid approved in Europe for visual interpretation of colour images and will be commercially available in selected European countries from early 2015. VIZAMYL™ is for diagnostic use only and should be used in conjunction with a clinical evaluation.
“Dementia is one of the biggest health and social challenges in the world and receiving marketing authorisation for VIZAMYL in the European Union (EU) demonstrates our continued commitment to helping to meet this challenge and support the diagnosis of Alzheimer’s disease” said Kieran Murphy, President and CEO, Life Sciences, GE Healthcare. “This approval will provide physicians in the EU with an important tool that may help them better assess specific patients who are being evaluated for Alzheimer’s disease and will also support further research into greatly needed disease modifying agents.”
Alzheimer's disease, the leading cause of dementia, can be challenging to diagnose, as many of the symptoms are similar to other causes of cognitive impairment1. When used in conjunction with a clinical evaluation, using VIZAMYL to detect the accumulation of beta amyloid in the brain may help to confirm an Alzheimer’s disease diagnosis and could potentially have an impact on earlier patient management, including the treatment of symptoms.
“VIZAMYL can help with diagnosis in certain individuals, providing patients and caregivers, along with their healthcare professionals, the opportunity to determine appropriate treatment options and plan for the future,” said Professor Philip Scheltens, Professor of Cognitive Neurology and Director of the Alzheimer Center at the VU University Medical Center, Amsterdam. “Because Alzheimer’s disease and dementia continue to be a major burden on healthcare and society, it is equally important that VIZAMYL will help support and guide further clinical research that is vital in order to develop disease modifying agents.”
Even though disease modifying agents are not yet available, new research released from the GE Healthcare-sponsored Value of Knowing Survey 20142 revealed an overwhelming demand to know about a neurological diagnosis in oneself or their loved ones, even in absence of a cure. The survey found that 74% of respondents would want to know their neurological disease diagnosis, even if there is no cure, and 81% would want to know the diagnosis if someone close to them had an incurable neurological disorder. The ‘Value of Knowing’ is a global survey of 10,000 adults across 10 countries exploring perspectives on incurable neurological disorders including Alzheimer’s (AD) and Parkinson’s (PD). The research was conducted by Millward Brown during June 2014 across 10 countries – Australia, Brazil, China, India, Indonesia, Japan, Russia, South Korea, UK and USA – with 1,000 nationally –representative adult respondents in each market.
The marketing authorisation for VIZAMYL was based on review of data from a series of Phase III clinical trials, including brain autopsy studies which showed high sensitivity and specificity for visual interpretation of flutemetamol (18F) PET images, using beta amyloid pathology as the standard of truth.3,4 Additionally, to instruct physicians in accurate interpretation of VIZAMYL images, GE Healthcare has developed an electronic reader training program (ETP), which, following approval, will be offered free of charge as online and in-person training in the European Union. Images should be interpreted only by readers who have completed the GE Healthcare electronic reader training program.
GE’S COMMITMENT TO IMAGING RESEARCH
VIZAMYL, flutemetamol (18F) is one component of a broad portfolio of investigational diagnostic solutions that GE Healthcare is currently developing in the neurology field. GE Healthcare is taking a comprehensive approach to understanding dementia and AD through its ongoing research to uncover the causes, risks, and physical effects of these diseases. GE Healthcare offers a broad portfolio of imaging resources including cyclotrons and chemistry systems to manufacture PET imaging agents, PET and MR scanners to scan patients, and is developing image analysis software to provide quantification, optimized visualization and reporting tools.
Additionally, GE Healthcare is collaborating with other companies in the pharmaceutical industry to assist in their development of the next generation of therapies. To that end, work is ongoing with potential partners in the industry to understand their strategic needs, and helping to provide imaging support for clinical trials of therapeutic agents.
ABOUT GE HEALTHCARE
GE Healthcare provides transformational medical technologies and services to meet the demand for increased access, enhanced quality and more affordable healthcare around the world. GE (NYSE: GE) works on things that matter - great people and technologies taking on tough challenges. From medical imaging, software & IT, patient monitoring and diagnostics to drug discovery, biopharmaceutical manufacturing technologies and performance improvement solutions, GE Healthcare helps medical professionals deliver great healthcare to their patients.
For our latest news, please visit http://newsroom.gehealthcare.com
1Alzheimer’s Association. What is Dementia Patient Information. Available at http://www.alz.org/what-is-dementia.asp. Last accessed August 2014.
2 Value of Knowing 2014. GE Healthcare. Data on file.
3 Ikonomovic M, Buckley C, Smith A et al. [18F]Flutemetamol injection PET images reflect brain amyloid levels. Data presented at Alzheimer's Association® International Conference 2012 (AAIC 2012), Vancouver, BC, Canada.
4 Wolk D, Gamez J, Sadowsky C et. al. Brain autopsy and in vivo cortical brain biopsy trials show a strong concordance between [18F]Flutemetamol PET and amyloid-β pathology. Poster presented at: 64th Annual Meeting of the American Academy of Neurology, April 21-28, 2012; New Orleans, LA.