CAESAREA, Israel--(BUSINESS WIRE)--Itamar Medical reported today, June 9th, 2014, that the new and upgraded model of the WatchPAT™, Itamar Medical's leading sleep apnea diagnostic device, has been cleared by the FDA. The FDA clearance came earlier than anticipated, and was announced at the Annual Meeting of the Associated Professional Sleep Societies, which took place in Minneapolis, MN, with 4800 sleep physicians and professionals attending the conference. The newest model has received a lot of hype from physician and sleep expert attendees.
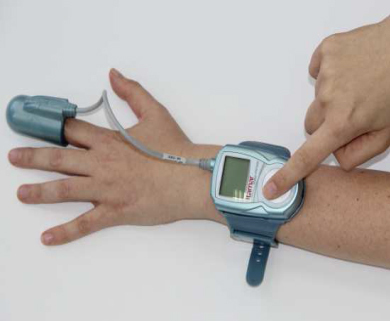
Within the WatchPAT™ Unified, the patented PAT® sensor will measure blood oxygen saturation in addition to the PAT® signal in a single Unified probe. In previous models of the WatchPAT™, the patient wore a watch-like device on the wrist with two finger sensors attached to it, one measuring the PAT® signal and one oximetry sensor measuring blood oxygen saturation. The newest model, which features a single unified finger probe (see picture), simplifies the test procedure, and improves reliability and user comfort while taking the test.
Mr. Gilad Glick, CEO of Itamar Medical, said: "“This is a substantial achievement for Itamar Medical. We expect our channels to the market, both through our direct sales force to the core sleep physicians and through our exclusive relationships in the cardiology space to benefit from this FDA cleared innovation. Itamar Medical continues to invest great efforts into its R&D with our goal to continuously lead the home sleep test market for diagnosing Sleep Apnea and sleep related breathing disorders, while keeping it simple and comfortable for the patient.”
Itamar Medical is a public medical technology company (TASE: ITMR) developing non-invasive medical devices using its proprietary PAT® signal (Peripheral Arterial Tone). Its two product lines: WatchPAT™, a medical-grade home device for diagnosis of sleep breathing disorders, and EndoPAT™, a non-invasive endothelial function assessment device used to improve risk-stratification and to personalize treatment in symptomatic and cardiovascular disease patients across all disease states.
The PAT® signal is measured non-invasively through the finger tips using a custom programmed finger sensor and signal processing algorithms developed by the Company, and validated in hundreds of scientific publications.