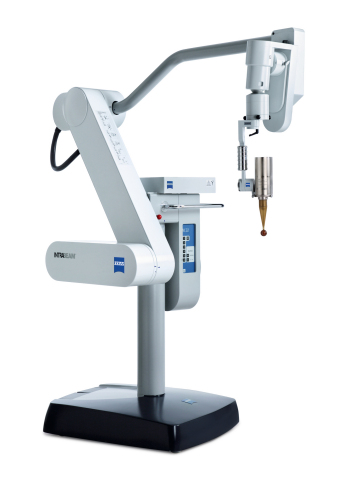
DUBLIN, Calif. & JENA, Germany--(BUSINESS WIRE)--"While our focus has been on intraoperative radiotherapy for breast cancer, we now also offer patented radiation technology for patients with skin or gastrointestinal tumors, or with metastases in the spine area," says Dr. Ludwin Monz, President and CEO of Carl Zeiss Meditec AG. With INTRABEAM radiotherapy, either the tumor itself, or the tumor margins, after removal of the tumor, are locally irradiated with a low energy 50 kV X-ray. "Patients who are potential candidates for this type of treatment receive radiation targeted directly to the diseased tissue with the dose and duration of the treatment adapted to the specific needs of each patient," Ludwin Monz continues.
In the past 14 years ZEISS INTRABEAM® has been utilized around the world for more than 8,000 patients, predominantly for breast cancer. With more than 200 installed systems, ZEISS is now the market and innovation leader in intraoperative radiotherapy for breast cancer. The world's largest study on partial breast irradiation, TARGIT-A, was conducted exclusively with INTRABEAM. The study included 3451 patients in 33 international centers, half of whom received external beam radiotherapy (EBRT) and half targeted intraoperative radiotherapy (TARGIT). The study compared ZEISS INTRABEAM to traditional whole breast irradiation in women 45 years and over with invasive ductal carcinoma. The TARGIT-A trial demonstrated that for a select group of patients targeted intraoperative radiotherapy with ZEISS INTRABEAM Spherical Applicators at the time of lumpectomy to be non-inferior to traditional EBRT.
With INTRABEAM, unlike traditional radiotherapy, a low-energy X-ray source is brought into direct contact with the tumor or tumor margin. This means that the affected tissue is irradiated with a high degree of precision, thus reducing irradiation to healthy tissues and organs. Due to the low X-ray energy, INTRABEAM does not require any complex radiation shielding measures and is even suitable for mobile use.
For non-melanoma skin cancers, a new radiotherapy treatment option with the INTRABEAM Surface Applicator is now available for patients, particularly those with high surgical risk or those requiring treatment on cosmetically relevant areas where surgery is not desired. Together with the new INTRABEAM Flat Applicator and the Needle Applicator, the benefits of INTRABEAM treatment – precise radiation dose targeted directly to the diseased area – can now be utilized for other types of cancer such as spinal cancers.
Carl Zeiss Meditec AG
Carl Zeiss Meditec AG (ISIN: DE0005313704) is one of the world’s leading medical technology companies. The company supplies innovative technologies and application-oriented solutions designed to help doctors improve the quality of life of their patients. It provides complete packages of solutions for the diagnosis and treatment of eye diseases, including implants and consumables.
The company creates innovative visualization solutions in the field of microsurgery. Carl Zeiss Meditec's medical technology portfolio is rounded off by promising, future-oriented technologies such as intraoperative radiotherapy. In fiscal year 2011/12 (ended 30 September) the company's around 2,400 employees generated revenue of just under EUR 862 million. Carl Zeiss Meditec headquarters are located in Jena, Germany.
The company has subsidiaries in Germany and abroad; more than 50 percent of its employees are based in the USA, Japan, Spain and France. The Center for Application and Research in India (CARIn) in Bangalore, India, and the Carl Zeiss Innovation Center for Research and Development in Shanghai, China, strengthen the company's presence in these fast-growing countries. Around 35 percent of Carl Zeiss Meditec shares are in free float. The remaining 65% are held by Carl Zeiss AG, one of the world’s leading groups in the optical and optoelectronic industries.
In the markets for Industrial Solutions, Research Solutions, Medical Technology and Consumer Optics, Carl Zeiss has contributed to technological progress for more than 160 years and enhances the quality of life of many people around the globe. Carl Zeiss AG, Oberkochen, is fully owned by the Carl Zeiss Foundation.
For more information, please go to: www.meditec.zeiss.com