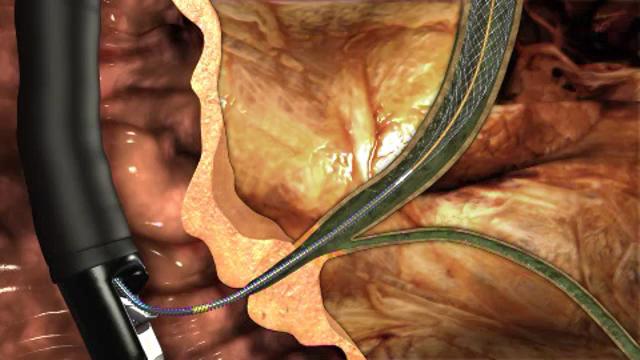
WINSTON-SALEM, N.C.--(BUSINESS WIRE)--The Food and Drug Administration has granted 510(k) clearance for the Evolution® Biliary Controlled-Release Uncovered Stent from Cook Medical. The biliary stent adds to Cook’s line of Evolution controlled-release stents for the gastrointestinal (GI) tract. It is the only delivery system that allows the stent to be deployed or recaptured as needed, putting control in the physician’s hands.
Approximately 35,500 metal biliary stents are placed each year1 to relieve symptoms associated with obstruction of the bile duct. The woven construction of the Evolution biliary stent is designed to maintain patency and specifically to aid in preventing stent migration after placement.
Cook’s family of Evolution stents has a delivery system that allows a physician to deploy or recapture the stent in equal increments by squeezing the trigger on the handle. A “point of no return” mark alerts the physician when the stent is deployed too far to be recaptured. The system also features Cook’s patent pending Flexor Plus™ technology and remains flexible yet firm enough to be navigated through difficult paths in the anatomy.
“We are thrilled with this addition to the Evolution family,” said Barry Slowey, global leader of Cook Medical’s Endoscopy division. “Now clinicians can experience the same precision and control throughout the entire GI tract, from the esophagus to the colon.”
The Evolution biliary uncovered stent received CE mark in the spring of 2012, and more than 1,200 stents have been placed in patients since then. The Evolution biliary will be available across the United States in July. The Evolution line of stents includes biliary, colonic, duodenal and esophageal options.
About Cook Medical
Since 1963 Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we are combining medical devices, biologic materials and cellular therapies to help the world's healthcare systems deliver better outcomes more efficiently. We have always remained family-owned so that we have the freedom to focus on what we care about: patients, our employees and our communities. Find out more at www.cookmedical.com, and for the latest news, follow us on Twitter and LinkedIn.
1 Millennium Research Group. US Markets for Gastrointestinal Endoscopy Devices 2010. Toronto, Canada: Millennium Research Group; 2010.