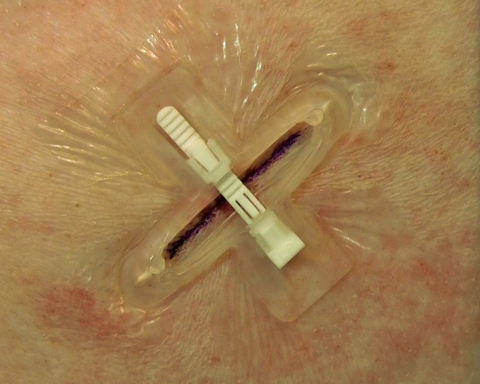
CAMPBELL, Calif.--(BUSINESS WIRE)--ZipLine Medical, an emerging medical device company that is developing a platform technology for noninvasive surgical skin closure designed to provide a suture-like outcome at the speed of staples, reported today that it has begun shipping products for commercial use.
“Traditionally, closure of large incisions has required sutures at the skin surface, a sometimes time-consuming process. Alternatively, staple closure saves time, but causes a worse final appearance of the wound. Now, with the ZipLine device, we can achieve both goals: better cosmesis and time savings,” said David C. Gorsulowsky, M.D., Dermatologic Surgeon and Associate Clinical Professor, University of California San Francisco. Dr. Gorsulowsky is one of the first commercial adopters of this new technology.
“Of course, the commercial launch of our products is a very significant milestone for ZipLine Medical,” said John Tighe, President and CEO. “Given that skin-closure is the common denominator of nearly all surgical procedures, ZipLine’s PRELOC™ technology platform for skin closure has extensive applicability across multiple medical specialties. Our initial target applications include pacemaker/ICD implant, spine and hip orthopedic, excisional skin biopsy and laceration closure,” said Tighe.
“Our goal is to invest in world-class entrepreneurial teams who are committed to achieving the commercialization of new technology over a condensed period of time. John Tighe and the ZipLine team are to be commended for their efforts and success,” said Ted Driscoll, Ph.D., Partner at Claremont Creek Ventures.
“We support passionate visionaries who are pursuing game-changing innovations at the onset of their company’s earliest stage. The commercialization of the ZipLine product family fills a critically important market need not only for enhanced convenience of both patients and doctors but also for best-in-class cosmetic results,” said Robert Siegel, a General Partner at XSeed Capital Management.
ZipLine Medical (www.ziplinemedical.com), headquartered in Campbell, Calif., is an emerging medical device company that has developed a platform technology — PRELOC™ — for noninvasive surgical skin closure via a simple, easy-to-learn and easy-to-use device designed for suture-like outcomes at the speed of staples.
NOTICE: ZipLine® devices are classified by the U.S. FDA as ‘Class I, Exempt.’ The Company has confirmation of FDA determination of this classification on file.