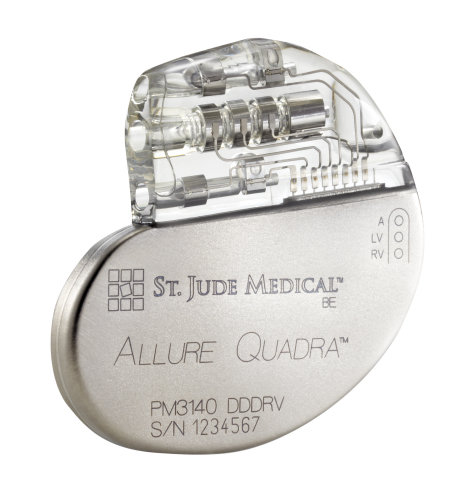
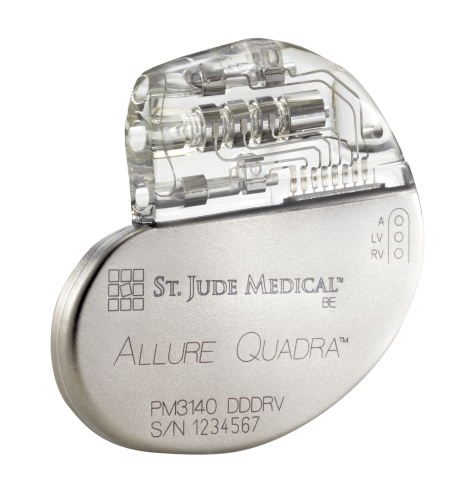
ST. PAUL, Minn.--(BUSINESS WIRE)--St. Jude Medical, Inc. (NYSE:STJ), a global medical device company, today announced CE Mark approval and European launch of its Allure Quadra™ Cardiac Resynchronization Therapy Pacemaker (CRT-P). This CRT-P system offers more pacing options to deliver higher success rates, as evidenced by robust clinical data. Allure Quadra CRT-P brings the quadripolar lead technology to the pacemaker market for the first time. Quadripolar leads allow for increased implant efficiencies, which clinical data indicates can result in fewer surgical revisions. The Allure family of devices also offer enhanced heart failure (HF) diagnostics, including CorVue™ Impedance Monitoring, for improved patient management.
“Adding quadripolar technology to the CRT-P platform moves us forward in the treatment of heart failure and allows us to expand the proven clinical benefits to a new patient population,” said Dr. Amir Zaidi, consultant cardiologist and devices lead at Manchester Royal Infirmary, England. “The new features and additional pacing options allow for optimal lead placement and provide the patient greater opportunities to respond to therapy, while reducing the need for re-intervention.”
The new platform of St. Jude Medical low-voltage devices includes the only CE Mark approved algorithm for identifying stroke risk. The ASSERT, or ASymptomatic AF and Stroke Evaluation in Pacemaker Patients and the AF Reduction Atrial Pacing Trial, was designed to determine whether the detection of arrhythmias using pacemaker-based diagnostics predicts an increased risk of stroke in elderly, hypertensive patients without any history of atrial fibrillation (AF). Results found that pacemaker patients who have no history of atrial tachycardia (AT) or atrial fibrillation (AF), but have device-detected arrhythmias, are approximately 2.5 times more likely to have a stroke than patients who do not have device-detected arrhythmias.
“Since its launch in 2010, the St. Jude Medical quadripolar CRT-D platform has allowed physicians to more effectively treat their patients and we expect the same results with our quadripolar CRT-P line,” said Eric S. Fain, M.D., president of the Implantable Electronic Systems Division at St. Jude Medical. “We believe these benefits result in a cost-effective solution that elevates the standard of care for heart failure patients in need of cardiac resynchronization therapy.”
Approximately one in 10 patients who receive a bipolar CRT system has complications that could require additional surgery. St. Jude Medical’s quadripolar technology allows for additional pacing configurations that provide physicians with options not available on traditional bipolar systems. These additional options help manage common pacing complications without exposing the patient to additional surgeries for lead repositioning.
Additionally, the Allure CRT-P device improves HF management with timely access to vital diagnostic data including CorVue™ Impedance Monitoring for earlier insight into HF progression, and Direct Trend™ Reports for a simplified look at all device data. The Allure Quadra CRT-P integrates the best-in-class quadripolar technology with multiple pacing configurations and TailoredTherapy™ features designed to enable physicians to address the dynamic challenges of HF by optimizing the quadripolar system at implant and follow-up.
Currently, heart failure results in more than 3.6 million hospitalizations per year in Europe, and related costs represent approximately two percent of overall health care expenditures.
Visit St. Jude Medical during The Heart Rhythm Society’s 34th Annual Scientific Sessions next week at booth #1003 for more information on our new platform of low-voltage devices with the quadripolar technology.
The Allure Quadra CRT-P device is currently not approved for use in the U.S. For additional information on our market-leading quadripolar technology, visit sjm.com.
About St. Jude Medical
St. Jude Medical develops medical technology and services that focus on putting more control into the hands of those who treat cardiac, neurological and chronic pain patients worldwide. The company is dedicated to advancing the practice of medicine by reducing risk wherever possible and contributing to successful outcomes for every patient. St. Jude Medical is headquartered in St. Paul, Minn. and has four major focus areas that include: cardiac rhythm management, atrial fibrillation, cardiovascular and neuromodulation. For more information, please visit sjm.com.
Forward-Looking Statements
This news release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that involve risks and uncertainties. Such forward-looking statements include the expectations, plans and prospects for the Company, including potential clinical successes, anticipated regulatory approvals and future product launches, and projected revenues, margins, earnings and market shares. The statements made by the Company are based upon management’s current expectations and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. These risks and uncertainties include market conditions and other factors beyond the Company’s control and the risk factors and other cautionary statements described in the Company’s filings with the SEC, including those described in the Risk Factors and Cautionary Statements sections of the Company’s Annual Report on Form 10-K for the fiscal year ended December 29, 2012. The Company does not intend to update these statements and undertakes no duty to any person to provide any such update under any circumstance.